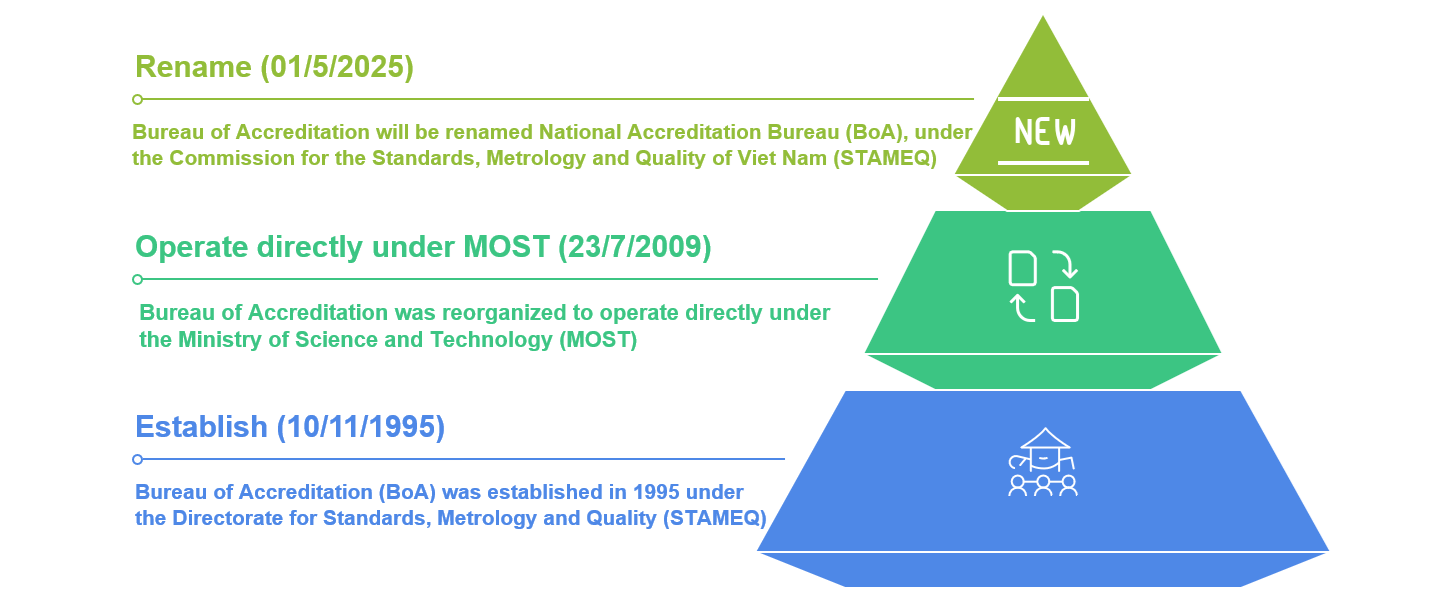
History of Establishment and Development

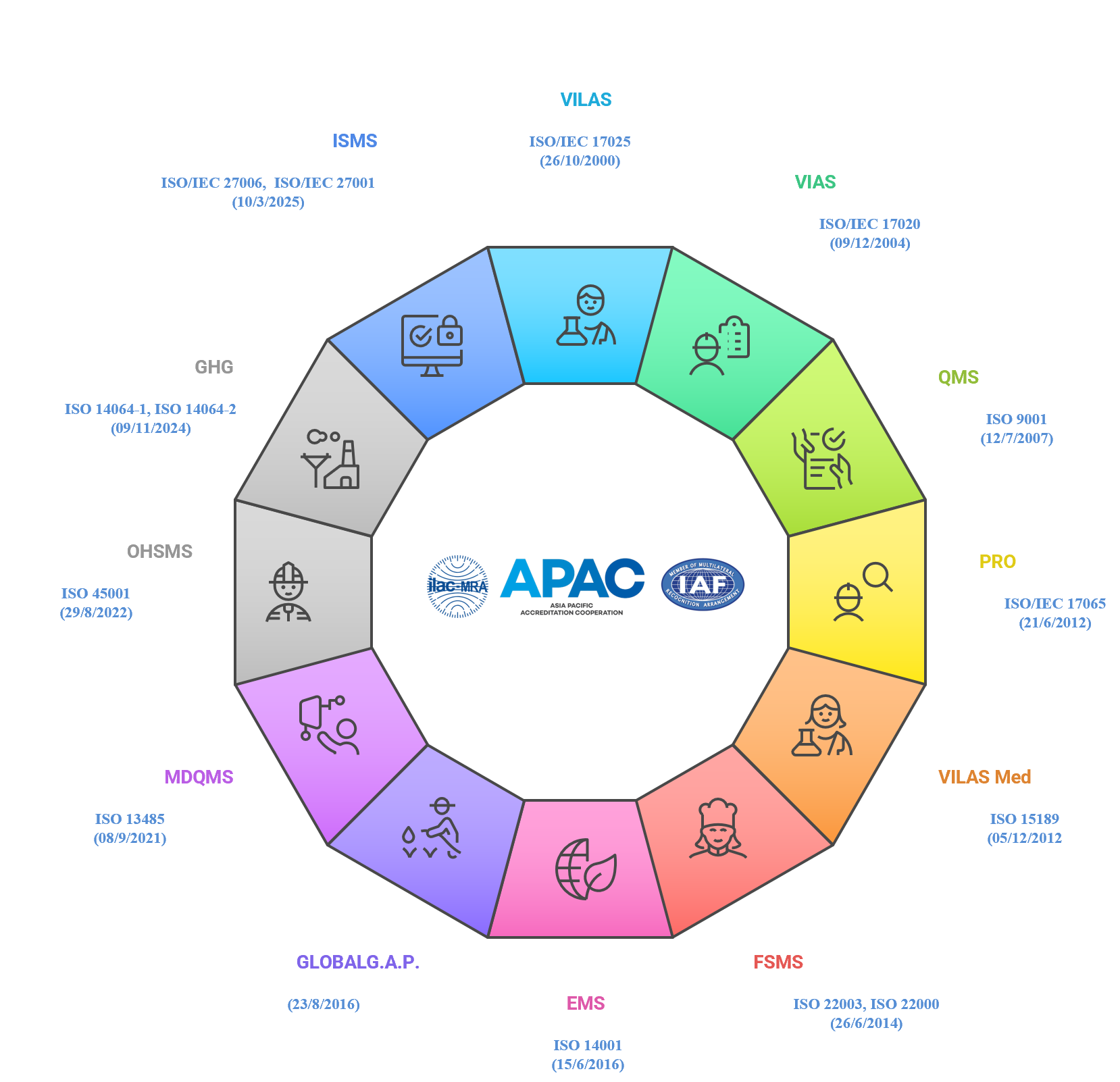
- Currently, BoA provides accreditation services for:
- Testing and calibration laboratories (VILAS);
- Inspection bodies (VIAS);
- Certification bodies, Validation and Verification bodies (VICAS);
- Medical laboratories (VILAS Med);
- Proficiency testing provider (VIPAS);
- Reference material producer (VIRAS);
- Laboratories/Facilities following GLP OECD Principles;
- Other conformity assessment bodies.
- In addition to accreditation activities, BoA also provides training services on accreditation standards and requirements for conformity assessment bodies.
- BoA is now a full member of international and regional accreditation organizations such as the:
- Asia Pacific Accreditation Cooperation (APAC);
- International Laboratory Accreditation Cooperation (ILAC);
- International Accreditation Forum (IAF).
- BoA is a signatory to the Mutual Recognition Agreement (MRA) of APAC and ILAC for the following programs:
- Testing and calibration laboratories (VILAS) since 2000;
- Inspection bodies (VIAS) since 2004;
- Medical laboratories (VILAS Med) since 2012.
- BoA is also a signatory of Mutual Recognition Agreement (MRA/MLA) to of APAC and IAF for the following certification programs:
- To date, BoA has granted accreditation across all provinces of Vietnam and in several other Asian countries (including China, Laos, Cambodia, Brunei, Philippines, etc.), covering nearly 2,000 laboratories, calibration laboratories, medical laboratories, inspection bodies, certification bodies, validation and verification bodies, proficiency testing providers, and reference material producers.
- BoA also maintains regular cooperation with several international accreditation bodies, including: KAB (Korea), GAC (Saudi Arabia), UKAS (United Kingdom), JAS-ANZ (Australia & New Zealand), ACCREDIA (Italy)…
